Clinical research on lentivirus as a gene transfer vector has begun
China Education Equipment Purchasing News: Lentivirus vector is based on the lentiviral genome and is constructed by replacing some genes with the desired target genes. The lentiviral vectors currently used are mostly modified from the HIV-1 genome. Compared with general retroviral vectors, lentiviral vectors have the ability to infect both dividing and non-dividing cells and have a wider host range. Lentiviral vectors can also effectively integrate foreign genes into the host chromosome, thereby achieving durable expression. In terms of infectivity, it can effectively infect various types of cells such as neuronal cells, liver cells, cardiomyocytes, tumor cells, endothelial cells, stem cells, etc., and rarely triggers the body's immune response. It can achieve good gene therapy effects and has a broad range of Application prospects.
Lentivirus (Lentivirus) is a type of retrovirus, which requires a relatively long incubation time, so it is called "Lentivirus", Lenti means slow in Latin. It includes human immunodeficiency virus (HIV), feline immunodeficiency virus (FIV), simian immunodeficiency virus (SIV), bovine immunodeficiency virus, etc. One of the most studied is the HIV-1 lentivirus.
With the in-depth study of lentiviral vectors, in order to improve the clinical safety of lentiviruses, the optimization of lentiviral vectors is also under constant discussion. The development of lentiviral vectors has gone through three stages. The first-generation lentiviral vector system is represented by a three-plasmid system. The cis-acting originals required for packaging, reverse transcription and integration of the HIV-1 genome during construction The sequences encoding trans-acting proteins are separated and constructed on three plasmid expression systems, namely packaging plasmid, envelope plasmid and vector plasmid. The packaging plasmid controls the expression of all viral structural genes except env under the action of the cytomegalovirus (CMV) promoter; the envelope plasmid encodes the vesicular stomatitis virus (VSV) G glycoprotein; the carrier plasmid Contains the gene of interest. Using these three plasmids to co-transfect packaging cells such as human embryonic kidney 293T cells, the lentiviral particles with only one infection capacity and no replication capacity can be harvested in the cell supernatant. The characteristics of the first generation lentiviral vector system are that when constructing three packaging plasmids, in order to reduce the possibility of producing viruses with replication ability, the homologous sequences between the three plasmids are reduced as much as possible, but the packaging plasmid still retains HIV Gene. The second-generation lentiviral vector system was improved on the basis of the first generation, and all accessory genes of HIV were deleted from the packaging plasmid. The removal of these accessory genes does not affect the virus titer and infectivity, while increasing the safety of the vector. The third-generation lentiviral vector system adds two additional safety features: one is the construction of its own inactivated lentiviral vector, that is, the 3 ′ LTR of the U3 region is deleted, so that the vector loses the HIV-1 enhancer and promoter sequences, even RNA cannot be transcribed in the presence of all viral proteins; second, the tat gene is removed and replaced with a heterologous promoter sequence, so that only 3 of the 9 lentiviral vectors in the original HIV genome are retained (gag, pol, and rev ). Therefore, the third-generation lentiviral vector system is more secure.
At present, lentivirus has been widely used in RNA interference research. Due to the poor transfection of some types of cell liposomes, the short half-life of shRNA transferred into cells, and the inhibition of gene expression by siRNA synthesized in vitro are usually short-lived, so their application is also greatly restricted. By constructing a vector that can express shRNA in vitro beforehand, and then packaging it into a lentiviral particle that can express shRNA, it can directly infect some difficult-to-handle cells, which is not only convenient and easy to implement, but also can achieve long-lasting suppression of gene expression.
Research on lentiviral vectors based on lentiviruses has developed to a certain level, so clinical research using lentiviruses as gene transfer vectors has begun. In laboratory and clinical studies, lentiviruses have particular advantages, especially those that can infect non-division Cells and immune response are small. Now clinical research on HIV-based lentiviral vectors has begun to be applied to humans.
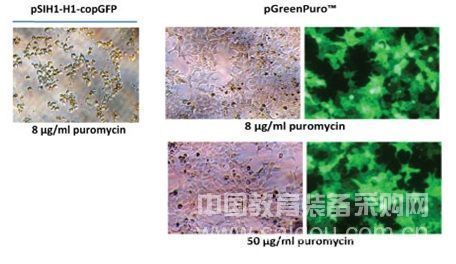
The lentiviral products provided by Jikai Gene to the market use a third-generation lentiviral vector system with higher safety performance. The packaging plasmid provides the necessary lentiviral genes to form the structural protein and packaging function of the virus particles; the envelope plasmid provides the package Membrane protein; vector plasmid not only expresses the gene of interest, but also provides packaging signals and cis-acting originals. The lentiviral titer that GK Gene provides to the market can reach 1E + 8 TU / mL, and it can infect target cells without concentration and purification. After lentivirus infects the target cells, it is screened with puromycin, or by green fluorescence emitted by GFP. Allows you to study the function of genes such as humans, mice and rats more conveniently and quickly.
We could make different size of Color Packaging Box and Paper Gift Box base on your design.we aer the China Hang Tag ,Printing Label,Cardboard Boxes,Paper Bags Manufacturer and Supplier.
Paper Gift Box
Paper Gift Box,Paper Box,Pretty Gift Boxes,Square Gift Box
Xing Hua Printing Factory , https://www.xinghuaprint.com